Overview
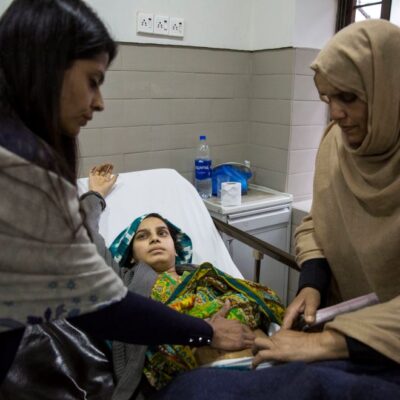
The WOMAN Trial was a randomised, double-blind, placebo-controlled trial that investigated the effects of early administration of tranexamic acid (TXA) on death, hysterectomy and other relevant outcomes in women with postpartum haemorrhage.

Trial background
Heavy bleeding after childbirth, or PPH, is the main cause of maternal death, killing tens of thousands of mothers every year. The WOMAN Trials have a simple aim; to ensure a safe childbirth for all women everywhere.
The premise for the WOMAN Trial came from the results of the 2010 CRASH-2 Trial, which showed that TXA reduces death due to bleeding after serious injury with no apparent increase in thrombotic events.
Treatments for PPH such as blood transfusion can be expensive, with blood stocks not always available and with varying levels of quality control. Although tranexamic acid is commonly used to reduce surgical bleeding, before the WOMAN Trial there had been no proper clinical trials to assess its value in treating excessive bleeding after childbirth. Therefore a large trial testing a simple, relatively cheap intervention like TXA could prevent deaths and morbidity associated with PPH.
Trial in numbers
20,060
Participants recruited
21
Recruiting countries
193
Hospitals
Results
The trial, which published its results in 2017, found that TXA could save the lives of mothers who would otherwise bleed to death after childbirth.
In the trial, death due to bleeding was reduced by about a third if the treatment was given within three hours. The trial also show that tranexamic acid reduces the need for surgery to control bleeding (laparotomy) by more than a third.
There were no side effects observed from the drug for either mothers or babies.
Full publication
Trial protocols
Get in touch
If you would like to find out more about WOMAN, please email us at ctu@lshtm.ac.uk.
Latest news
Read all the latest evidence, news and opinions.